High temperature stress can severely limit crop productivity, particularly at critical stages of plant development. We have established that the cytosolic molecular chaperone, Hsp101, is essential for the ability of plants to survive high temperature stress. Hsp101 is a member of the Hsp100/ClpB chaperone proteins, which are hexameric ATPases believed to function in disaggregation of denatured proteins. Significantly, plants are the only higher eukaryotes known to express this type of chaperone, indicating studies of Hsp101 should lead novel insights into mechanisms of stress tolerance. We are using genetic and molecular approaches to investigate Hsp100/ClpB proteins, including studies of both cytosolic Hsp101 and chloroplastic ClpB-p, in the model plant Arabidopsis thaliana. The ultimate goal is to define mechanistically how these chaperones influence plant growth, development, stress tolerance and productivity. In one approach we have isolated extragenic suppressors of an Hsp101 mutant in Arabidopsis in order to define essential substrates or partner proteins of Hsp101, and as a way to uncover components of other mechanisms required for thermotolerance. We are also using affinity methods in transgenic plants to isolate Hsp101 associated proteins.
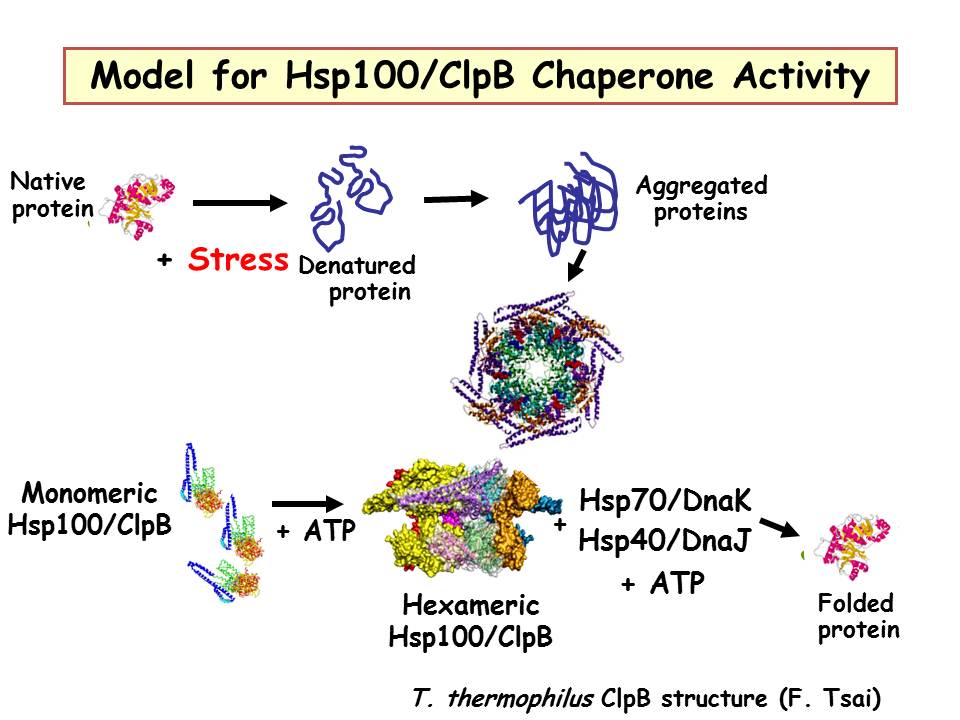
The HSP100/ClpB proteins can disaggregate denatured proteins with the help of Hsp70, cochaperones and ATP. Hsp70 acts upstream and potentially downstream of Hsp100/ClpB. Substrate proteins enter the central channel of the chaperone (shown here as a model from the T. Thermophilus structure in two views).