Research
The secretory pathway in humans manages one-third of the proteome by overseeing the folding, modification, and assembly of its clients within the endoplasmic reticulum (ER) before directing them to their destinations. The ER is temporally and spatially organized to support the efficient maturation of its diverse clients. Clients generally enter co-translationally through translocons where an assembly line of factors eagerly awaits the arrival of the vulnerable nascent chains. As the newly formed chain undergoes folding and progresses deeper into the ER, it undergoes multiple quality control checks to evaluate its structural integrity and readiness for departure at ER exit sites. Proteins that haven't acquired their native conformation are held back to afford them additional opportunities for achieving the correct structure. Once ample chances for proper folding have been utilized, non-native cargo is marked for degradation, ensuring the preservation of cellular homeostasis.
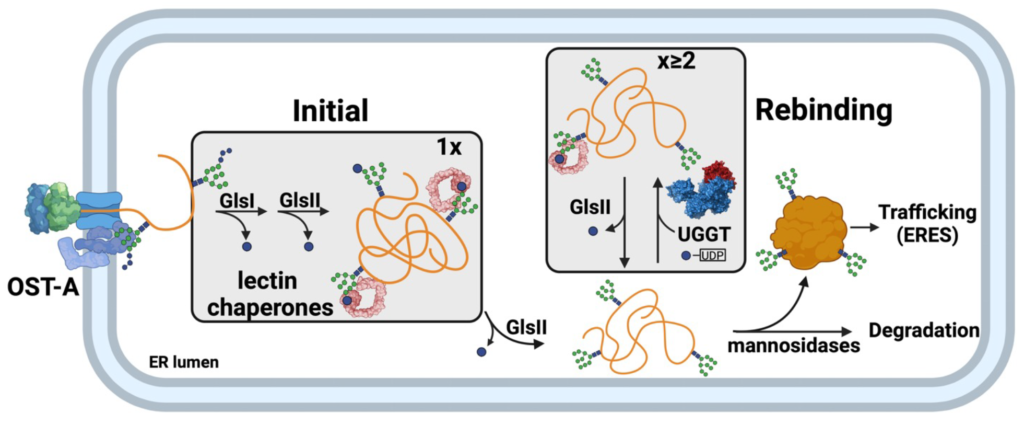
Over 80% of secretory pathway clients receive N-glycans upon entering the ER. These glycans act as protein folding, quality control and sorting tags. They serve to recruit protein folding and quality control factors, which aid glycoproteins in their maturation and trafficking. After their attachment, the N-glycan is remodeled by a series of ER glycosidases and transferases such that the maturation status of the protein is encoded by its N-glycan composition or what we refer to as the ER glyco-code. The main focus of the Hebert lab is deciphering the glyco-code and the mechanism by which it controls the folding, quality control and trafficking of glycosylated clients as they traverse the secretory pathway. We utilize glycosylated clients whose misfolding is closely linked to a variety of disease states including the serpins alpha-1-antitrysin (COPD and liver cirrhosis), antithrombin (thrombosis) and neuroserpin (neurodegenerative diseases), as well as a number of viral envelop glycoproteins. Our work is helping to better understand the molecular choreography in the ER for thousands of glycosylated secretory pathway clients, including many for which misfolding is the root of disease states. We employ a variety of cell biological, biochemical and molecular biological approaches to study the maturation, quality control and degradation of glycoproteins using cell-free assays, isolated organelles and live cells.