S-Nitrosoglutathione Reductase (GSNOR)
Nitric oxide (NO) is a short-lived, endogenously produced radical gas that acts as a signaling molecule in all higher organisms. However, the complex chemistry of NO results in multiple transformations in biological systems, complicating our understanding of the mechanism of NO effects. Directly and via chemical transformations, NO can accomplish not only signaling, but also act as both an antioxidant by quenching other radical reactions, or pro-oxidant through production of reactive nitrogen species (RNS). Regulatory effects of NO are mediated through protein modification, including heme nitrosylation, tyrosine nitration, S/Cys nitrosylation and glutathiolation, which are the result of direct reaction with NO or with its products. Effects of NO in plants are diverse. NO has been described to regulate aspects of plant defense responses, stomatal movement , flowering and germination. Wounding and a number of abiotic stresses are reported to induce NO, and NO has been linked to salicylic acid and jasmonic acid signaling.
NO also reacts with the major intracellular antioxidant glutathione (GSH) to form S-nitrosoglutathione (GSNO), which can then transfer its NO group to other cellular thiols to form S-nitrosothiols (SNOs). This NO adduct has been proposed to be a significant player in NO regulatory mechanisms, particularly in S-nitrosylation of proteins. The role of GSNO in transferring NO to protein thiols implies that metabolism of GSNO is a major branch of NO metabolism that impacts many regulatory processes. Increasing numbers of proteins are reported to be reversibly nitrosylated in plants and other organisms. This modification may be one of the major effectors of NO-mediated responses and is sometimes referred to as “the new phosphorylation”.
We are studying the enzyme S-nitrosoglutathione reductase (GSNOR), originally identified in plants and other organisms as formaldehyde dehydrogenase (FALDH), a type III alcohol dehydrogenase. Using mutants of GSNOR in Arabidopsis (hot5 mutants), we have shown that GSNOR is ciritical to nitric oxide homeostasis. The hot5 mutants are sensitive to heat stress, most likely because they cannot control production of RNS. We are studying the other phenotypes of hot5 to understand how NO metabolism impacts specific plant functions, including fertility. We are also interested in identifying the major NO-modified proteins in plants.
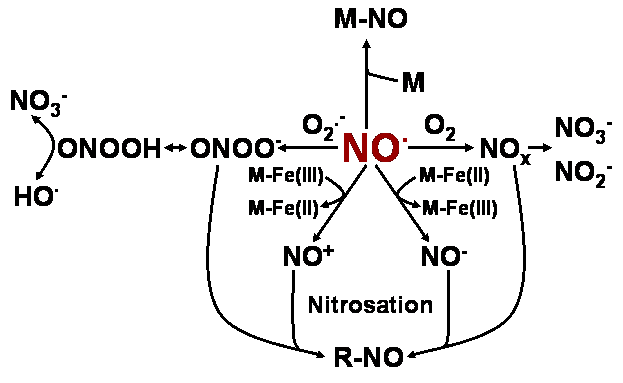
Chemistry of nitric oxide (NO) in biological environments. M–NO, iron–nitrosyl complexes; M, heme- or iron- proteins; M–Fe(II)/Fe(III), Fe(II)/Fe(III)- metalloproteins; NOx = NO2., N2O3 and N2O4; R–NO, nitrosylated proteins.

Generalized pathway of NO and GSNO production. GSNO can be metabolized by GSNOR, or act as an NO or GS donor as shown.